Anodizing is a process for altering the chemistry of the surface of other substrates as well as metals. It helps prevent corrosion, improves aesthetics and resists scratching. It is among the longest-lasting finish options that are available. Anodizing is a process that can be applied to various materials, but in this case, we’ll look at aluminum. These eight questions will assist in determining the benefits of anodizing as a process that is practical as well as beautiful.
1. What do you know if Aluminum Anodized?
For the purpose of preparing aluminum to be anodized the surface is first cleaned and rinsed. The surface is afterwards, it is immersed in a tub of an electrolytic solution such as sulfuric acid. Electrolytes are an electrically conductive substance with a large amount of negative and positive ions it wishes to exchange.

An electric current is applied positive to aluminum, creating the “anode” and the negative charge is placed on the plates within the electrolyte. The current generated by the electric circuit triggers positive ions be drawn towards the plates with negative charges and negative ions to be drawn to the positive anode, which is the piece made of aluminum.
2. How do you define a Barrier? anodizing?
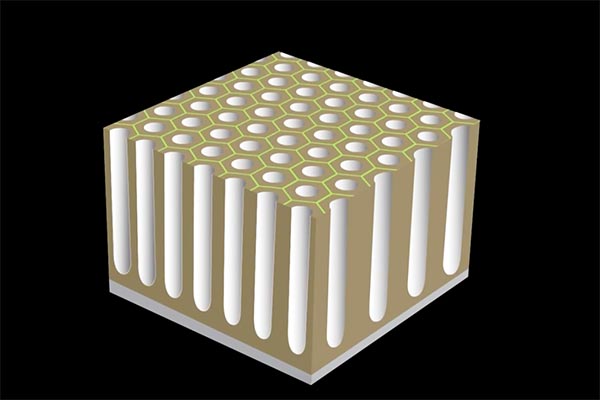
The electrochemical reaction causes pores to develop on the surface of the aluminum when the excess positive ions are released. The pores form a geometrically regular pattern that begins to break down to the surface. The aluminum that is on the surface is combined with positively charged 2ions to form aluminum oxide. This is referred to as an insulating layer, or protecting against chemical reactions that occur at these spots.
If current is continued to be applied in the near future, the relatively weak and active areas of the pores continue to expand in the surface. Eventually forming columns-like hollow structures.
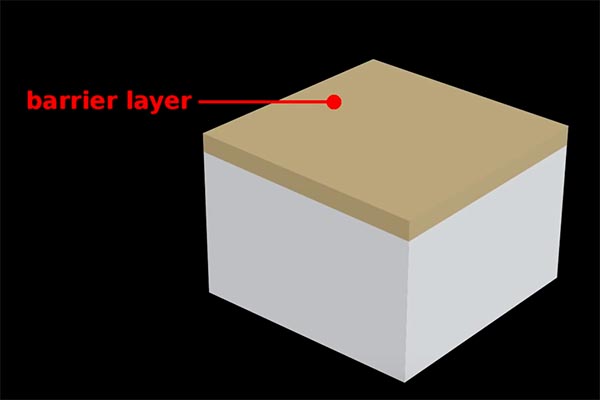
The longer the time that the current is applied, the deeper the depth of the columns. For normal non-hard coatings the depth could range from 10-microns. When the level is attained and there isn’t any color requirement the process ceases and the surface is protected by simply rinsing it in water. You will be left with an extremely hard natural aluminum oxide coating which is resistant to chemical attacks and is extremely resistant to scratches. It is classified as 9 of 10 in the Mohs hardness scale, which is that it is second only to diamond.
3. What is the definition of Hard Anodizing?
Hard anodizing, also known as Type III. Offers greater protection against corrosion and resists wear in harsh conditions or when working with mechanical parts that experience lots of friction. This is accomplished by maintaining the current of electricity until the thickness of the pores is greater than 10 microns all the way up to 25 microns, or even higher. It takes longer and costs more, yet results in a better outcome.
4. Does Aluminum Need Corrosion Protection?
While aluminum isn’t rusty but it does degrade in the presence of oxygen, a process known as the process of oxidation. What exactly is the term “oxidation? It’s simply the word used to mean to react with oxygen. Oxygen is extremely reactive and readily forms compounds with other elements. After exposure of aluminum the atmosphere , it immediately creates a layer with aluminum oxide over the entire surface and this gives an amount of protection against further corrosion.

However, aluminum has to withstand more than pure water and air. Acid rain as well as salt water and other contaminants could attack the weaknesses of the passivation of the surface. Even the most modern alloys can change in reaction to environmental exposure. It can range from surface discoloration to up to failure due to mechanical damage.
5. How can color be added to the Metal Anodizing?
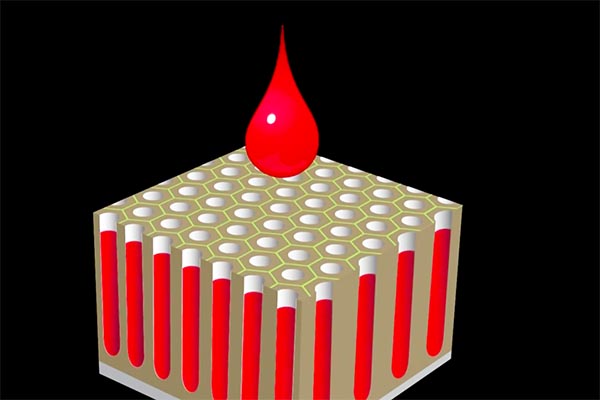
Colored aluminum is the thing that most of us envision when we imagine anodizing. This is the true genius of this method. The lovely, stable pores that are etched into the surface make it ideal for the introduction of hues and pigments.
The pigment fills all spaces that are empty up to the surface, which is eventually sealed permanently. That’s the reason the colors that are anodized are extremely durable They aren’t scratched away from the surface since in reality, the colors are deep and are only eliminated by grinding the surface.
6. Why does anodized aluminum always have that distinctive metallic Sheen?
Anodized aluminium has an attractive “metallic” appearance. This is due to two causes. One is because of uniform electrochemical etching process that leaves a rough surface left behind. The more pores there are will be, the more rough the surface will appear, but the colors will be more robust.
Second, light that hits the surface interacts partially with the colorant, and also with the non-colored metal on the top.
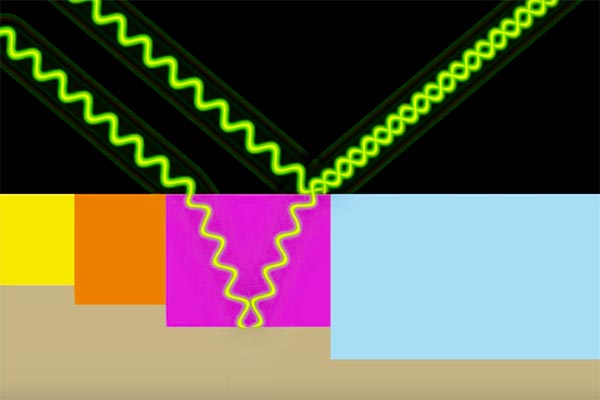
Thus the beam of light which bounces off to your eyes will actually comprise two distinct wavelengths that interact as they reflect from different surfaces. This creates the distinct sparkle of the anodizing process on aluminum.
7. Other materials than aluminum can be anodized?
Yes. Anodizing can also be used with titanium, magnesium and even conductors in plastics. It’s low-cost, reliable, and extremely sturdy. It’s the reason it’s often employed in architectural fittings because it’s beautiful and nearly impervious to consequences of weathering.
8. Why is it impossible to anodize the entire Part?
Anodizing is a process whereby a piece is submerged in a number of baths containing chemicals. In order to hold a part in place, it is dependent on it being fixed to the hanger of some sort to stop it from falling down to at the base inside the tank. Anytime the holding fixture comes into contact with the piece, the area is blocked and the anodizing chemicals will not function correctly. This is why it’s important to create a spot on your component that can be used to hold but isn’t affected in terms of appearance.
Is Anodizing Right For You?
If you call us to request an estimate and review of your project. We’ll be in a position to provide assistance on the various finishing services we offer for quick protoypes and low-volume manufacturing. Our experts will assist you find the right solution that is best suited to your budget, timeline to market, and desired outcomes. We’re ready to get started!

